Abstract
Background: Patients (pts) with relapsed-refractory (R-R) acute lymphoblastic leukemia (ALL) have a poor prognosis. The combination of low-intensity chemotherapy with mini-hyper-CVD (mini-HCVD) and inotuzumab has shown encouraging results. The aim of this study is to evaluate whether the sequential addition of blinatumomab to mini-HCVD-inotuzumab with lower doses of weekly inotuzumab might further improve outcomes.
Methods: Pts with Philadelphia chromosome-negative B-cell ALL were included. Odd cycles of mini-HCVD consisted of cyclophosphamide (150 mg/m2 every 12 h on days 1-3), vincristine (2 mg flat dose on days 1 and 8), and dexamethasone (20 mg on days 1-4 and days 11-14) without anthracyclines. Even cycles consisted of cytarabine (0.5 g/m2 given every 12 h on days 2 and 3) and methotrexate (250 mg/m2 on day 1). During the first 4 courses, pts received rituximab when CD20 expression was ≥20%, and intrathecal chemotherapy. Initially, inotuzumab was administered on day 3 of the first 4 cycles at the dose of 1.3-1.8 mg/m2 in course 1, followed by 1.0-1.3 mg/m2 in subsequent courses. Pts received maintenance therapy with POMP, consisting of 1 year of monthly prednisone 50 mg/d for 5 days and vincristine at 2 mg every month, along with 3 years of 6-mercaptopurine 50 mg twice daily and weekly oral methotrexate 10 mg/m2. An amendment to the protocol was made after the inclusion of 67 pts to add 4 cycles of blinatumomab after 4 cycles of the combination mini-HCVD + inotuzumab. Inotuzumab was given on days 2 and 8 at the dose of 0.6 and 0.3 mg/m2 in course 1, respectively, followed by days 2 and 8 at the dose of 0.3 and 0.3 mg/m2 in subsequent courses; blinatumomab was continuously infused over 28 days every 42-day cycle for 4 cycles. Maintenance therapy was reduced to 12 courses of POMP with 1 course of blinatumomab after each 3 courses of POMP for a total of 4 courses. The decision to undergo allogeneic stem cell transplantation (ASCT) was based on the discretion of the treating physician after discussion with the patient.
Results: Between February 2013 and July 2021, 112 pts were treated on study, including 45 pts with mini-HCVD + inotuzumab + blinatumomab. Baseline characteristics and outcomes are summarized in Table 1. The median follow-up is 41 months (range, 3-112). Before the study amendment, 67 pts were treated: 38 (57%) in salvage(S)1, and 29 (43%) in S2+. Nineteen pts (28%) had received prior ASCT. After the amendment, additional 45 pts were treated: 42 (93%) in S1, and 3 (7%) in S2+; 3 (7%) pts had prior ASCT.
The overall response rate (ORR) was 83% (CR, 63%) in the entire cohort, 76% (CR, 60%) pre-amendment, and 93% (CR, 67%) post-amendment. Among 91 responders evaluable for measurable residual disease (MRD), 76 pts (82%) achieved MRD negativity by multicolor flow cytometry, with higher rates of MRD negativity in S1 (89%). The rates of MRD negativity before and after the amendment were 82% and 85%, respectively. Overall, 53 (47%) pts underwent ASCT: 29 (43%) pts pre-amendment and 24 (53%) post-amendment.
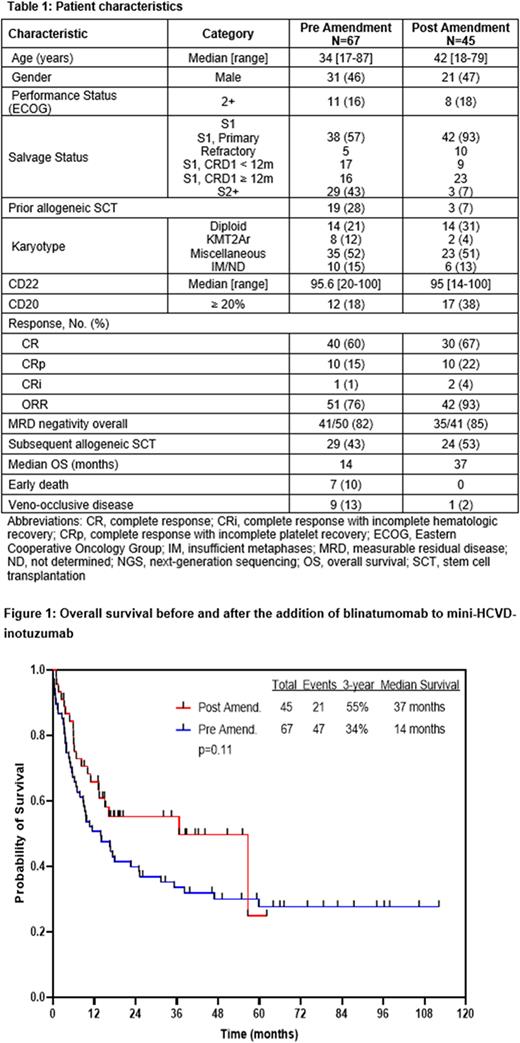
The median overall survival (OS) for the entire cohort was 17 months, with a 3-year OS rate of 41%. After the amendment, the addition of blinatumomab resulted in superior median OS (37 months vs 14 months) and 3-year OS rates (55% versus 34%) (Figure 1). Pts in S1 had better survival compared with those in S2+, with 3-year OS of 51% and 17%, respectively (P=0.001). In a 2-month landmark analysis among pts in S1, the median OS was 57 months in those who received blinatumomab compared with 31 months in those who did not. Overall, pts who achieved MRD negativity had higher 3-year OS rate of 55% compared to 10% in pts who were MRD-positive at best response (P=0.0001).
Survival with mini-HCVD + inotuzumab +/- blinatumomab compared favorably to that of inotuzumab monotherapy: 17 months vs 6 months; P<0.0001. A 3-month landmark analysis among responders showed similar 3-year OS rates between pts who underwent subsequent ASCT and those who did not (55% vs 48%, respectively).
Veno-occlusive disease (VOD) was observed in 10 of the 112 pts (9%), and its incidence was reduced from 13% with single dose of inotuzumab to 2% with fractionated dose schedule (P=0.056).
Conclusion: The addition of blinatumomab to mini-HCVD-inotuzumab, along with a lower weekly dose of inotuzumab, further improves the outcomes of pts with R-R ALL. These adjustments reduced the rate of VOD and improved survival. Long-term survival was similar regardless of subsequent ASCT.
Disclosures
Jabbour:Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Short:Amgen: Consultancy, Honoraria; Novartis: Consultancy; Pfizer: Consultancy; AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding. Jain:Novalgen: Research Funding; Takeda: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte Corporation: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Newave: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pfizer: Research Funding; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; Medisix: Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; Mingsight: Research Funding; TransThera Sciences: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria; Dialectic Therapeutics: Research Funding; Loxo Oncology: Research Funding; Servier Pharmaceuticals LLC: Research Funding; ADC Therapeutics: Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support. Sasaki:Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka Pharmaceuticals: Honoraria. Ravandi:AstraZeneca: Consultancy; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Novartis: Consultancy; Syos: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding. Kebriaei:Pfizer: Consultancy; Amgen: Research Funding; Ziopharm: Research Funding; Jazz: Consultancy; Kite: Consultancy. Konopleva:AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy. Garcia-Manero:AbbVie: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Astex: Consultancy, Honoraria, Research Funding; Curis: Honoraria, Research Funding; Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Aprea: Honoraria. Champlin:Johnson &Johnson: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; Actinium: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Cell Source Inc.: Research Funding. Kadia:JAZZ: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Research Funding; Servier: Consultancy; AstraZeneca: Research Funding; Astellas: Research Funding; Amgen: Research Funding; cellenkos: Research Funding; Astex: Honoraria; cyclacel: Research Funding; Ascentage: Research Funding; Regeneron: Research Funding; BMS: Consultancy, Research Funding; Iterion: Research Funding; PinotBio: Consultancy; Delta-Fly: Research Funding; Glycomimetics: Research Funding; Genentech: Consultancy, Research Funding; Genfleet: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. Takahashi:Illumina: Honoraria; Mission Bio: Honoraria; Ostuka Pharmaceuticals: Honoraria; GSK: Consultancy; Celgene/BMS: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy; Agios: Consultancy. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Kantarjian:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; ImmunoGen: Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.